Imagine getting a heads-up about diabetes risk more than a decade before you’d normally be diagnosed. That’s the promise behind a new AI system trained on continuous glucose monitor data.
A CGM (continuous glucose monitor) is a small wearable sensor, often placed on the upper arm, that checks your glucose all day and night. Instead of one lab value every so often, it captures a rolling “movie” of your glucose, including peaks, dips, and overnight patterns.
“12 years early” matters because it shifts the story from treating diabetes to preventing it. In this post, I’ll break down what the research says, how the model works in plain terms, what it could mean for care teams and product builders, and what I’d question before calling it “early diagnosis.” If you’re an AI builder, a clinician, or just curious, this is written for you. If I were publishing this, I’d add: a hero photo of a CGM on an arm (near the top), a simple timeline graphic (near the research claim), and a CGM waveform example (near the “signals” section).
What the new research claims: diabetes risk from one week of glucose patterns
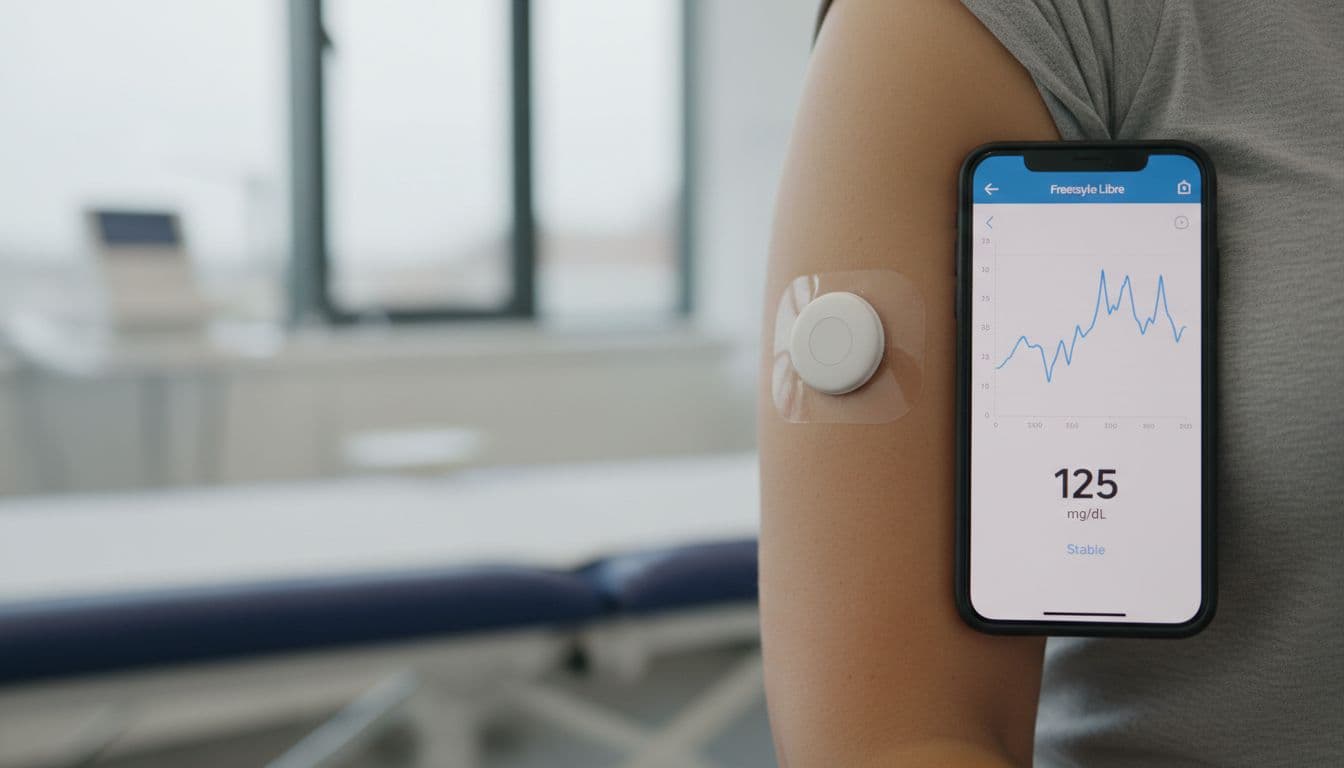
The headline claim is simple to say and hard to ignore: an AI model called GluFormer can estimate long-term diabetes risk using roughly one week of CGM readings, projecting risk as far as 12 years out.
This isn’t based on a single number like fasting glucose or HbA1c. It’s based on patterns. That matters because plenty of people look “fine” on standard labs, especially early on. Yet their day-to-day glucose behavior might still show subtle stress signals, like big meal spikes or slow returns to baseline.
The paper was published in Nature in January 2026, and it frames GluFormer as a foundation model for CGM data, meaning it can learn general patterns from large volumes of glucose traces and apply them to multiple prediction tasks. If you want the primary source, I’d start with the Nature article on the CGM foundation model.
Who built it, what data it learned from, and why that dataset matters
From the reporting and the study materials, the work is led by Prof. Eran Segal’s group at the Weizmann Institute of Science, with key contributors (including Guy Lutsker) and collaboration with NVIDIA and Pheno.AI.
The big reason this dataset matters is boring in a good way: scale and follow-up. The model was trained on long-term, real-world CGM data tied to later health outcomes, not just short lab snapshots. The materials describe a dataset on the order of 14,000 people, including many who were not diagnosed with diabetes at the time of monitoring. That mix is important, because early prediction only works if the model has seen lots of “not yet sick” patterns that later turned into disease.
How it connects to other AI and CGM breakthroughs (quick reality check)
I don’t treat GluFormer as a one-off miracle. I see it as part of a fast-moving trend: CGMs are becoming common, and AI is getting better at squeezing meaning out of time-series health data.
What’s different across projects is the goal. Some models try to predict near-term glucose response to meals. Others aim to estimate things like insulin resistance. Still others focus on earlier detection of autoimmune changes for Type 1 risk. Multiple groups are finding signal in glucose dynamics, but they’re not all solving the same problem, and they’re not all validated the same way.
How a “glucose transformer” model can forecast risk years ahead
At a high level, GluFormer treats CGM data like a sequence. If you’ve worked with language models, the idea will feel familiar.
 A language model reads words in order and learns which patterns tend to come next. A glucose transformer reads glucose points in order and learns which patterns tend to show up before certain outcomes (like future diabetes). It’s not “thinking” about glucose, it’s recognizing shapes, timing, and repetition across many people.
A language model reads words in order and learns which patterns tend to come next. A glucose transformer reads glucose points in order and learns which patterns tend to show up before certain outcomes (like future diabetes). It’s not “thinking” about glucose, it’s recognizing shapes, timing, and repetition across many people.
What I like about this framing is that it moves beyond averages. Two people can have the same average glucose and still look very different over a week.
If you want the technical deep dive, including how the model was trained and evaluated, the most direct reference is the arXiv preprint “From Glucose Patterns to Health Outcomes”.
What signals can hide inside CGM data (beyond fasting glucose and A1C)
When I look at CGM traces, I think of them like ocean waves. The average water level matters, but the waves can still tell you a storm is coming.
Here are glucose signals that can carry meaning even when labs look normal:
- Post-meal spikes (how high glucose jumps after eating)
- Recovery time (how long it takes to come back down)
- Overnight stability (is it flat, drifting up, or bouncing)
- Day-to-day variability (consistent days vs wild swings)
- Repeating patterns (similar spikes at similar times, across days)
These patterns can reflect early insulin resistance or other metabolic strain. They’re not a diagnosis on their own, and they can be distorted by sleep loss, stress, illness, or medications.
What the output might look like in practice (risk score, alerts, and next steps)
In a clinic or product setting, I don’t expect a model like this to shout “you will get diabetes.” I expect something more like a calibrated risk score with a time horizon, plus guidance on follow-up.
 A realistic “what happens next” flow could look like:
A realistic “what happens next” flow could look like:
- Confirmatory labs: HbA1c, fasting glucose, and sometimes an oral glucose tolerance test
- Lifestyle coaching: meals, protein and fiber timing, walking after meals, sleep habits
- Medication discussion (case-by-case): for some patients at high risk, a clinician may consider evidence-based prevention options
From a UX angle, the most important design choice is how you explain risk without causing panic. A high-risk flag should come with clear steps, not a scary notification.
What this means for prevention, care teams, and AI product builders
The best-case outcome here isn’t a fancy dashboard. It’s time.
If a model can spot elevated risk years earlier, prevention becomes more targeted. Primary care teams can focus attention where it’s most needed. Digital health programs can personalize coaching instead of sending generic advice. Researchers can recruit better cohorts for prevention trials.
If I had to summarize the practical upside in one line, it’s better triage.
Here are the use cases I think will show up first:
- Earlier prevention conversations in primary care, before labs cross a threshold
- Smarter CGM-based coaching that adapts to your patterns, not your averages
- More efficient screening programs (employers and insurers are already experimenting with CGMs)
- Trial recruitment for lifestyle and prevention studies, using consistent risk signals
If you’re building on top of CGM data, I’d treat this as a template for what “foundation models for health time series” can do, and a warning that validation will make or break adoption.
Where it could help first: screening, coaching, and personalized prevention
I’d bet on programs where the main goal is behavior support, not diagnosis. A week of CGM data is short enough to be practical, and long enough to capture routines like weekday meals and weekend changes.
The biggest win is that it buys years. If you can reduce risk before glucose control breaks, you can avoid a lot of downstream harm, both personal and financial.
How I would evaluate a diabetes prediction model before trusting it
When I see a “predicts diabetes 12 years early” claim, I don’t start with the ROC curve. I start with real-world questions:
- External validation: does it work on new health systems and new devices?
- Subgroup performance: does it hold across age, sex, and ethnicity?
- Calibration: does “20% risk” actually mean 20 out of 100 people?
- False alarms vs missed cases: who gets worried for no reason, and who slips through?
- Clinical utility: does it change outcomes, or just generate alerts?
- Pattern-level explainability: can it point to “frequent late-night rises” or “slow post-meal recovery” in human terms?
- Monitoring for drift: do results change as CGM adoption expands to new populations?
Then I ask the security questions, because CGM data is sensitive. Wearables, apps, cloud storage, and third-party analytics create a long chain of risk if it’s not locked down.
Limits, risks, and the questions I would ask before calling it “early diagnosis”
Risk prediction isn’t diagnosis. That sounds obvious, but it’s where messaging often goes off the rails.
Here are the pitfalls I watch for:
Selection bias is a big one. People who wear CGMs aren’t a random slice of the population. Data quality is another. Sensors fail, users calibrate incorrectly, and gaps happen. Behavior changes matter too, people often eat “better” during tracking weeks, which can hide typical spikes. Confounders show up everywhere: stress, poor sleep, infection, steroids, and menstrual cycles can all shift glucose.
There’s also the human factor. A risk alert can create anxiety, even if the model is right on average. Responsible tools pair risk with context and a plan.
For a practitioner-friendly summary of the research direction and claims, you can also see NVIDIA’s overview of GluFormer and health outcome prediction. For general diabetes risk guidance, I still point people to CDC and NIH resources, even when the AI looks impressive.
Privacy, consent, and data ownership with wearable glucose data
CGM data isn’t just numbers. It’s a behavioral timeline: timestamps, meal patterns (sometimes inferred), sleep windows, exercise effects, and routines. Even “de-identified” health time series can be re-identified in some settings.
 My baseline best practices are simple:
My baseline best practices are simple:
- Collect the minimum data needed for the task
- Encrypt data in transit and at rest
- Keep strict access logs and audits
- Limit retention, delete when it’s no longer needed
- Be plain about consent, and explain how the model uses the data
If a product can’t explain data use in a way a normal person understands, it’s not ready.
Where I land on this
One week of CGM data revealing long-term diabetes risk sounds almost unreal, but the underlying idea makes sense: patterns carry information that averages throw away. If models like GluFormer keep validating across populations, they could push care toward prevention years earlier than today’s workflow allows.
If you’re worried about diabetes, ask your clinician about risk testing and what follow-up makes sense for you. If you’re building these systems, my ask is simple: validate hard, message carefully, and treat privacy like a core feature, not a footnote.